

| New Experiments | |
|
I designed some new experiments to investigate some of the issues raised during the work for the RSC competition. Here are the experiments.... |
|
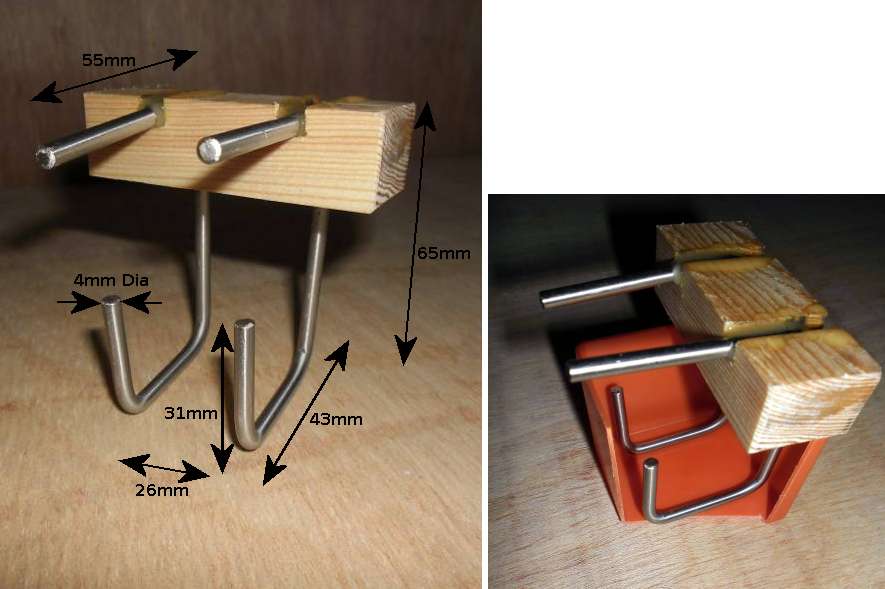
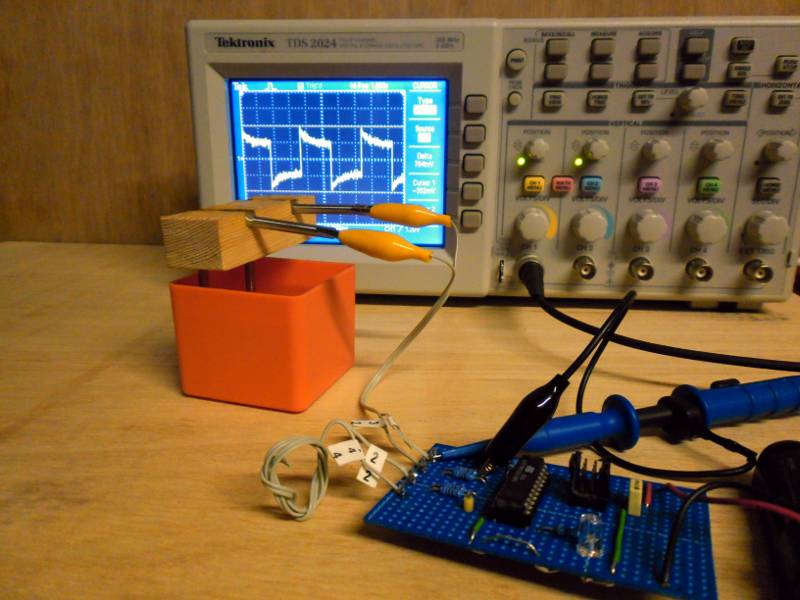
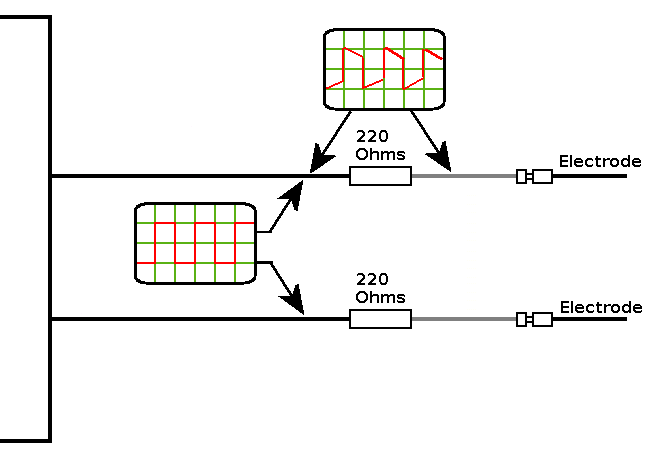

| Equipment 8 - Freezing De Ionised Water | |
|
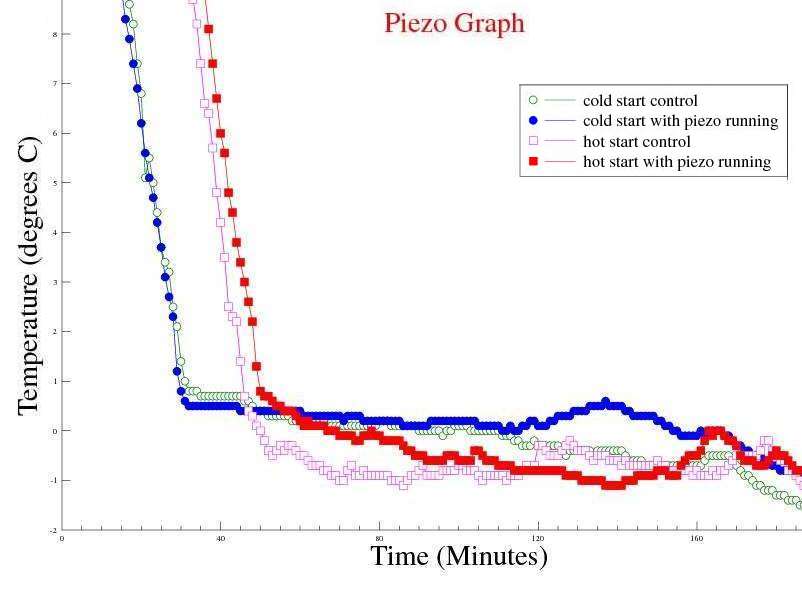
Objective: Use De Ionised Water rather than tap water and compare the results for: (1) Freezing De Ionised Water from cold. (2) Freezing De Ionised Water from Boiling. (3) Freezing De Ionised Water from cold, after treating with the HIVE , using inert electrodes for 30 minutes. Predictions: I would expect to see the untreated cold water sample take longer to freeze, the boiling water sample freeze faster than the untreated cold water and the water treated with the HIVE freeze in the shortest time. |
|

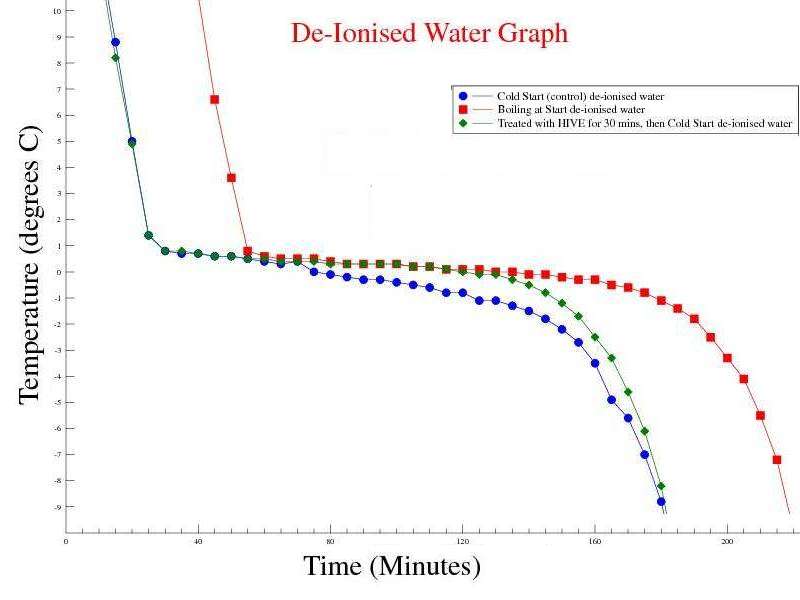
| Experiment 8 Conclusion | |
|
Observations: The Mpemba effect was not seen when using de ionised water - i.e. boiling water took longer to freeze than cold water. Issues raised by the observations: The Mpemba effect may rely on dissolved minerals and / or gasses in the water sample. |
|
| Equipment 9 - Freezing Bottled Mineral Water | |
|
Objective: Use Bottled Still Mineral Water rather than tap water or De Ionised Water and compare the results for: (1) Freezing Bottled Mineral Water from cold. (2) Freezing Bottled Mineral Water from Boiling. (3) Freezing Bottled Mineral Water from cold, after treating with the HIVE , using inert electrodes for 30 minutes. Predictions: I would expect to see the untreated cold water sample take longer to freeze, the boiling water sample freeze faster than the untreated cold water and the water treated with the HIVE freeze in the shortest time. |
|

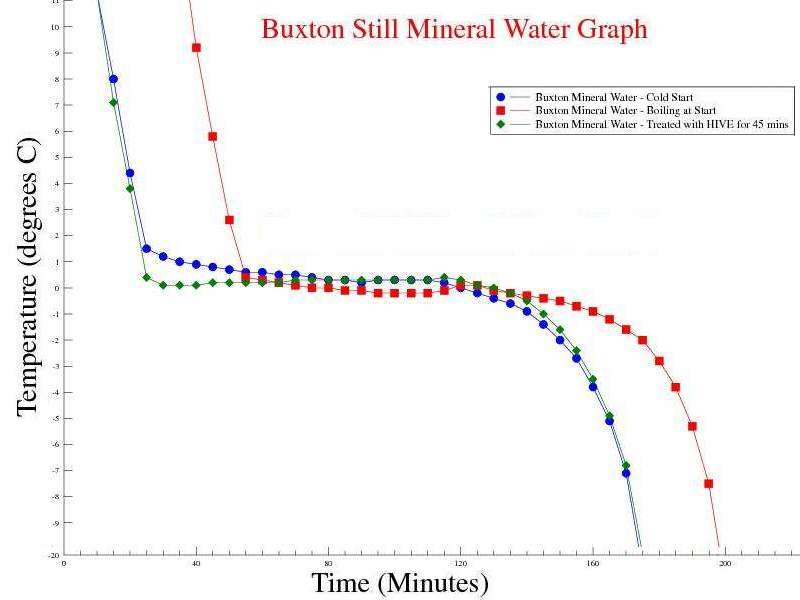
| Experiment 9 Conclusion | |
|
Observations: The Mpemba effect is still not visible, but the time to freeze hot water is faster than De ionised water. Issues raised by the observations: Maybe the concentration of minerals in the sample affects the time for hot water to freeze. Our tap water is in a hard water area (>200mg/l Calcium), whereas the Buxton sample has 55mg/l Calcium and De Ionised water has virtually no dissolved Calcium. 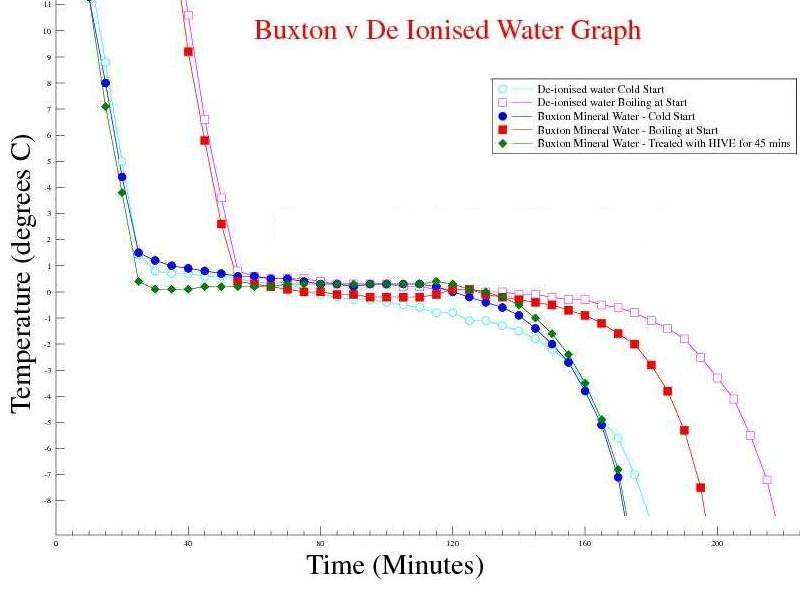
Buxton Mineral Water v Di Ionised Water. buxton_v_di_water_graph.gle = the GLE script to graph the above data sets. |
|